Calorimetry Lab Independent Variable . Measure the amount of heat involved in frostbite. Ca (s) + 2 h 2 o (l) →. Calculating the enthalpy change of reaction, hrfrom experimental data. in this lab, we will be investigating the endothermic and exothermic qualities of salt solutions by dissolving various salts into water and monitoring the. Some were variable and some were constant. To do so, the heat is exchanged with a. as part of this lab, you will: the lab procedure involves several factors, listed below. Heat is defined as thermal energy flowing from an object at a higher temperature to one. you will utilize a calorimeter to measure the energy absorbed or released in the following chemical reaction: calorimetry is used to measure amounts of heat transferred to or from a substance. measurement of an enthalpy change. a calorimeter is a device used to determine heat flow during a chemical or physical change. calorimetry is the science of measuring heat flow.
from www.studocu.com
measurement of an enthalpy change. Ca (s) + 2 h 2 o (l) →. a calorimeter is a device used to determine heat flow during a chemical or physical change. Measure the amount of heat involved in frostbite. the lab procedure involves several factors, listed below. as part of this lab, you will: in this lab, we will be investigating the endothermic and exothermic qualities of salt solutions by dissolving various salts into water and monitoring the. Heat is defined as thermal energy flowing from an object at a higher temperature to one. To do so, the heat is exchanged with a. you will utilize a calorimeter to measure the energy absorbed or released in the following chemical reaction:
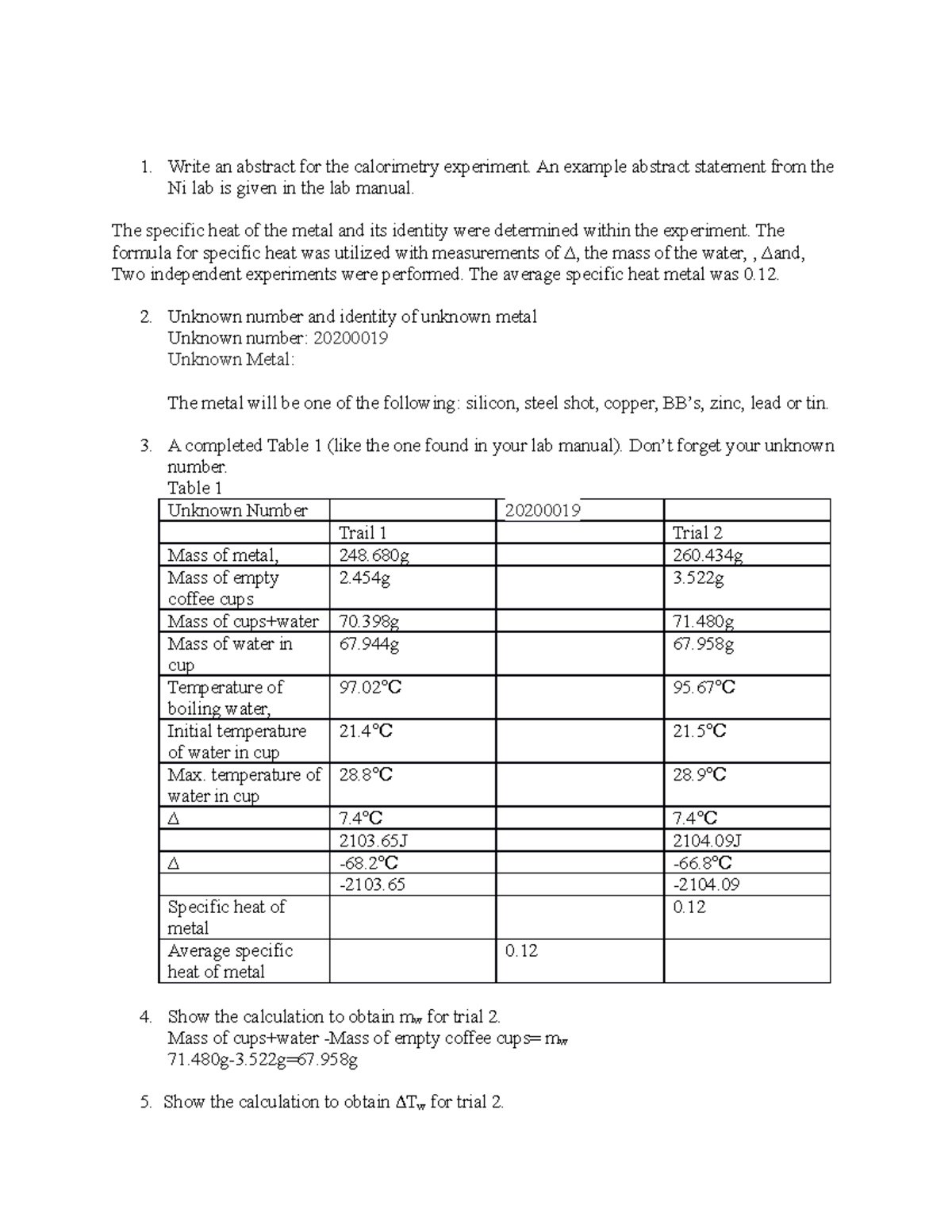
Calorimetry Lab Report Write an abstract for the calorimetry experiment. An example abstract
Calorimetry Lab Independent Variable you will utilize a calorimeter to measure the energy absorbed or released in the following chemical reaction: you will utilize a calorimeter to measure the energy absorbed or released in the following chemical reaction: To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a substance. measurement of an enthalpy change. Measure the amount of heat involved in frostbite. a calorimeter is a device used to determine heat flow during a chemical or physical change. Some were variable and some were constant. in this lab, we will be investigating the endothermic and exothermic qualities of salt solutions by dissolving various salts into water and monitoring the. as part of this lab, you will: the lab procedure involves several factors, listed below. Heat is defined as thermal energy flowing from an object at a higher temperature to one. Ca (s) + 2 h 2 o (l) →. calorimetry is the science of measuring heat flow. Calculating the enthalpy change of reaction, hrfrom experimental data.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Lab Independent Variable Heat is defined as thermal energy flowing from an object at a higher temperature to one. in this lab, we will be investigating the endothermic and exothermic qualities of salt solutions by dissolving various salts into water and monitoring the. calorimetry is the science of measuring heat flow. Calculating the enthalpy change of reaction, hrfrom experimental data. To. Calorimetry Lab Independent Variable.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle and Structure of Calorimetry Lab Independent Variable as part of this lab, you will: a calorimeter is a device used to determine heat flow during a chemical or physical change. Some were variable and some were constant. calorimetry is the science of measuring heat flow. the lab procedure involves several factors, listed below. calorimetry is used to measure amounts of heat transferred. Calorimetry Lab Independent Variable.
From www.studypool.com
SOLUTION Student exploration calorimetry lab vocabulary calorie calorimeter joule specific heat Calorimetry Lab Independent Variable Calculating the enthalpy change of reaction, hrfrom experimental data. Ca (s) + 2 h 2 o (l) →. you will utilize a calorimeter to measure the energy absorbed or released in the following chemical reaction: calorimetry is the science of measuring heat flow. Some were variable and some were constant. a calorimeter is a device used to. Calorimetry Lab Independent Variable.
From studylib.net
Calorimetry Experiment and Practice Problems for Virtual Lab Key Calorimetry Lab Independent Variable the lab procedure involves several factors, listed below. To do so, the heat is exchanged with a. Ca (s) + 2 h 2 o (l) →. Calculating the enthalpy change of reaction, hrfrom experimental data. Measure the amount of heat involved in frostbite. Some were variable and some were constant. in this lab, we will be investigating the. Calorimetry Lab Independent Variable.
From www.pinterest.com
Calorimetry Lab Report Science diagrams, Thermodynamics, How to find out Calorimetry Lab Independent Variable calorimetry is used to measure amounts of heat transferred to or from a substance. Ca (s) + 2 h 2 o (l) →. calorimetry is the science of measuring heat flow. measurement of an enthalpy change. Heat is defined as thermal energy flowing from an object at a higher temperature to one. you will utilize a. Calorimetry Lab Independent Variable.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Docsity Calorimetry Lab Independent Variable Calculating the enthalpy change of reaction, hrfrom experimental data. Heat is defined as thermal energy flowing from an object at a higher temperature to one. you will utilize a calorimeter to measure the energy absorbed or released in the following chemical reaction: To do so, the heat is exchanged with a. as part of this lab, you will:. Calorimetry Lab Independent Variable.
From saylordotorg.github.io
Calorimetry Calorimetry Lab Independent Variable To do so, the heat is exchanged with a. calorimetry is the science of measuring heat flow. in this lab, we will be investigating the endothermic and exothermic qualities of salt solutions by dissolving various salts into water and monitoring the. Calculating the enthalpy change of reaction, hrfrom experimental data. Ca (s) + 2 h 2 o (l). Calorimetry Lab Independent Variable.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Lab Independent Variable in this lab, we will be investigating the endothermic and exothermic qualities of salt solutions by dissolving various salts into water and monitoring the. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. Calculating the enthalpy change of reaction, hrfrom experimental data. calorimetry. Calorimetry Lab Independent Variable.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity Calorimetry Lab Independent Variable To do so, the heat is exchanged with a. Measure the amount of heat involved in frostbite. the lab procedure involves several factors, listed below. measurement of an enthalpy change. Heat is defined as thermal energy flowing from an object at a higher temperature to one. calorimetry is the science of measuring heat flow. Some were variable. Calorimetry Lab Independent Variable.
From exozspczc.blob.core.windows.net
Calorimeter Experiment Introduction at Jose Evans blog Calorimetry Lab Independent Variable calorimetry is the science of measuring heat flow. Calculating the enthalpy change of reaction, hrfrom experimental data. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. a calorimeter is a device used to determine heat flow during a chemical or physical change. . Calorimetry Lab Independent Variable.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry Lab Independent Variable calorimetry is the science of measuring heat flow. as part of this lab, you will: you will utilize a calorimeter to measure the energy absorbed or released in the following chemical reaction: To do so, the heat is exchanged with a. Some were variable and some were constant. the lab procedure involves several factors, listed below.. Calorimetry Lab Independent Variable.
From answermagicmatney.z21.web.core.windows.net
Coffee Cup Calorimetry Experiment Calorimetry Lab Independent Variable the lab procedure involves several factors, listed below. To do so, the heat is exchanged with a. as part of this lab, you will: Calculating the enthalpy change of reaction, hrfrom experimental data. Some were variable and some were constant. Ca (s) + 2 h 2 o (l) →. Heat is defined as thermal energy flowing from an. Calorimetry Lab Independent Variable.
From www.studocu.com
Expt25 Calorimetry lab report Expt25 Calorimetry Data A. Specific Heat of a Metal Unknown Calorimetry Lab Independent Variable as part of this lab, you will: calorimetry is the science of measuring heat flow. Calculating the enthalpy change of reaction, hrfrom experimental data. Heat is defined as thermal energy flowing from an object at a higher temperature to one. calorimetry is used to measure amounts of heat transferred to or from a substance. Some were variable. Calorimetry Lab Independent Variable.
From www.chegg.com
Solved label each variable in the given calorimeter Calorimetry Lab Independent Variable Ca (s) + 2 h 2 o (l) →. in this lab, we will be investigating the endothermic and exothermic qualities of salt solutions by dissolving various salts into water and monitoring the. as part of this lab, you will: calorimetry is the science of measuring heat flow. Heat is defined as thermal energy flowing from an. Calorimetry Lab Independent Variable.
From www.studocu.com
Calorimetry Prelab assignments Calorimetry Objective The purpose for this lab is to Calorimetry Lab Independent Variable Measure the amount of heat involved in frostbite. Calculating the enthalpy change of reaction, hrfrom experimental data. in this lab, we will be investigating the endothermic and exothermic qualities of salt solutions by dissolving various salts into water and monitoring the. calorimetry is used to measure amounts of heat transferred to or from a substance. measurement of. Calorimetry Lab Independent Variable.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Lab Independent Variable measurement of an enthalpy change. Measure the amount of heat involved in frostbite. you will utilize a calorimeter to measure the energy absorbed or released in the following chemical reaction: calorimetry is used to measure amounts of heat transferred to or from a substance. Calculating the enthalpy change of reaction, hrfrom experimental data. Some were variable and. Calorimetry Lab Independent Variable.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Lab Independent Variable Ca (s) + 2 h 2 o (l) →. calorimetry is used to measure amounts of heat transferred to or from a substance. Calculating the enthalpy change of reaction, hrfrom experimental data. Heat is defined as thermal energy flowing from an object at a higher temperature to one. calorimetry is the science of measuring heat flow. Some were. Calorimetry Lab Independent Variable.
From www.youtube.com
Unit 8 Food Calorimetry Lab YouTube Calorimetry Lab Independent Variable Some were variable and some were constant. measurement of an enthalpy change. Measure the amount of heat involved in frostbite. Heat is defined as thermal energy flowing from an object at a higher temperature to one. in this lab, we will be investigating the endothermic and exothermic qualities of salt solutions by dissolving various salts into water and. Calorimetry Lab Independent Variable.